JINGYE PHARMACEUTICAL
| News detail |
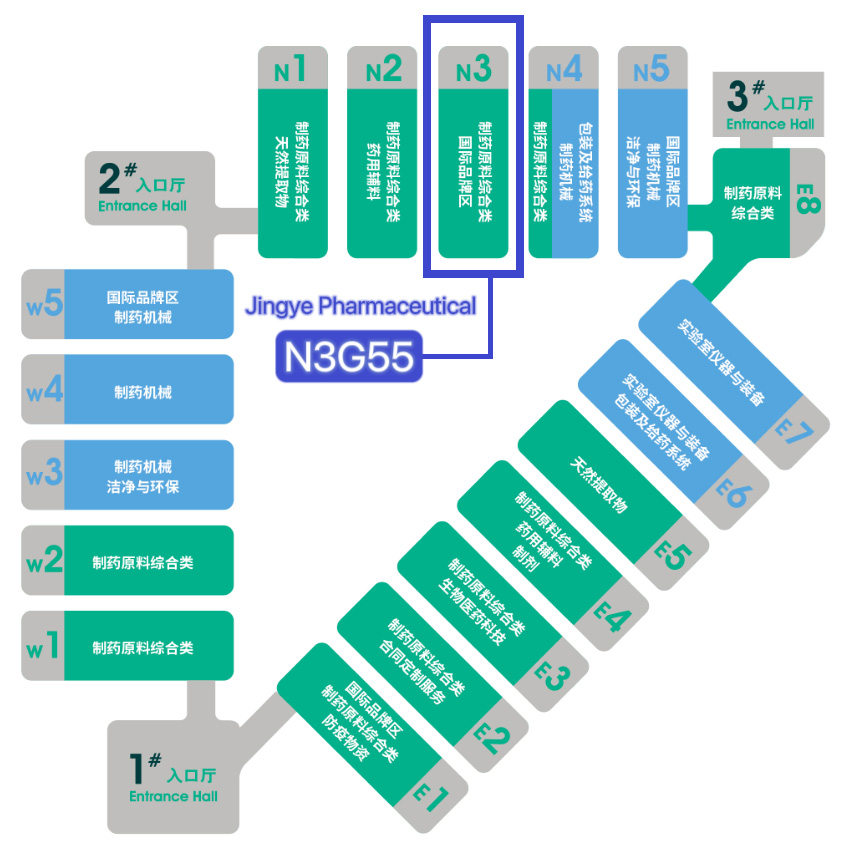
CPHI China2023 Jiangsu Jingye Pharmaceutical cordially invites you to meet us
Time: 2023-06-13



Scan it
Mobile version
Copyright(C)2024, Jiangsu Jingye Pharmaceutical Co., Ltd. All Rights Reserved. Supported by Sunsirs ChemNet Toocle Copyright Notice 备案序号:苏ICP备2024061058号